For that reason it is important, that you only use certified pyrogen-free equipment (e.g. tubes, vials, disposable tips, micro titre plates, ...), otherwise you could get false positive results. You also should qualify (in a documented way) that none of your equipment has any inhibiting or enhancing properties. Also all of the sample-types have to be examined on inhibition /enhancement.
Pharmalab is presently designed for the chromogenic LAL test method.
Principle of the test
 The endotoxin of gram-negative bacteria catalyses the activation of a proenzyme in the Limulus Amoebocyte Lysate (LAL). The rate of activation is controlled by the quantity of present endotoxin. The activated enzyme catalyses the cleavage of p-nitroaniline (pNA) (yellow) from the colourless substrate Ac-Ile-Glu-Ala-Arg-pNA.
The endotoxin of gram-negative bacteria catalyses the activation of a proenzyme in the Limulus Amoebocyte Lysate (LAL). The rate of activation is controlled by the quantity of present endotoxin. The activated enzyme catalyses the cleavage of p-nitroaniline (pNA) (yellow) from the colourless substrate Ac-Ile-Glu-Ala-Arg-pNA.
Measurement is performed photometrically at 405 nm.
Test methods
Endpoint
 After a defined reaction time, cleavage from the substrate is stopped by adding acetic acid, and the pNA formed is measured photometrically at 405 nm. The yellow colour is proportional to the endotoxin present.
After a defined reaction time, cleavage from the substrate is stopped by adding acetic acid, and the pNA formed is measured photometrically at 405 nm. The yellow colour is proportional to the endotoxin present.
The endotoxin content of the sample is determined using a calibration curve plotted using a standard endotoxin dilution series on each plate.
Kinetic
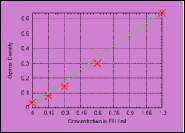 A kinetic reader automatically monitors the development of a yellow colouration (= substrate cleavage) over a specific time. The time taken to develop a yellow colouration of defined intensity (reaction time) is inversely proportional to the endotoxin present. The defined intensity of the yellow colouration which, on being reached, establishes the reaction time, is the Onset-OD or alternatively Delta m-OD. The test is concluded when the lowest standard has reached the Onset-OD. The endotoxin content of the sample is determined using a calibration curve plotted using a standard endotoxin dilution series on each plate.
A kinetic reader automatically monitors the development of a yellow colouration (= substrate cleavage) over a specific time. The time taken to develop a yellow colouration of defined intensity (reaction time) is inversely proportional to the endotoxin present. The defined intensity of the yellow colouration which, on being reached, establishes the reaction time, is the Onset-OD or alternatively Delta m-OD. The test is concluded when the lowest standard has reached the Onset-OD. The endotoxin content of the sample is determined using a calibration curve plotted using a standard endotoxin dilution series on each plate.

Das Pharmalab System LAL
- is a "web-based" application and handles all concerns of the LAL-test
- is 21 CFR part 11 compliant
- allows comprehensive user management
- contains a complete sample and task management
- executes all calculations
- does all reportings
- supports electronic signature
- leads you through manual test procedures
- controlles the micro plate readers
- triggers the automated methods of the TECAN Genesis 100/8 workstation
- is fully audit trailed
- has got various administration tools
When to buy a robot
 The decision for buying a robot primarily depends on the amount of samples you have to test.
The decision for buying a robot primarily depends on the amount of samples you have to test.
We recommend that you buy a robot, when you have more then 5000 samples per year. Also if your results differ from test to test, an automated workstation could solve many of reproduction issues.
The main advantage of a robot is of course its reproducibility, its efficiency and capacity.
The robot works fully automated; just load all equipment, samples and reagents and start the method.

